Regulatory affairs officers


60 ECTS
2025-2026

1 year

The Master 2 International Regulation of Digital Medical Devices focuses on the regulatory framework for Digital Medical Devices throughout their life cycle. Cross-disciplinary and international in scope, this course enables students to acquire expertise in the medical device regulatory sector, and more specifically in digital medical devices.
Objectives
- Train specialists in international regulatory affairs for medical devices, particularly digital medical devices.
- Develop students' ability to adapt to the scientific, industrial and societal ecosystem of the medical device industry.
- Provide students with a high level of knowledge for their specialization in the field of digital healthcare
- Promote the employability of graduates through contemporary academic training, internships and work-study programs.
- Offer interactive teaching based on innovative pedagogical practices: learning Lab, inverted course, interactive seminar, digital campus, interviews with professionals in the field, project-based learning, etc.
Target audience
The Master 2 is open to both initial and sandwich training.
- L3 science students with a solid grounding in biological or chemical sciences (including physical chemistry)
- DFASP2 or DFASM3 students (Diplôme de formation approfondie en sciences médicales et pharmaceutiques)
- Interns in Pharmacy or Medicine
- Holders of a diploma in pharmacy, medicine or veterinary medicine
- Candidates benefiting from validation of prior learning
- IT engineers
- Master's graduates in computer science
Course
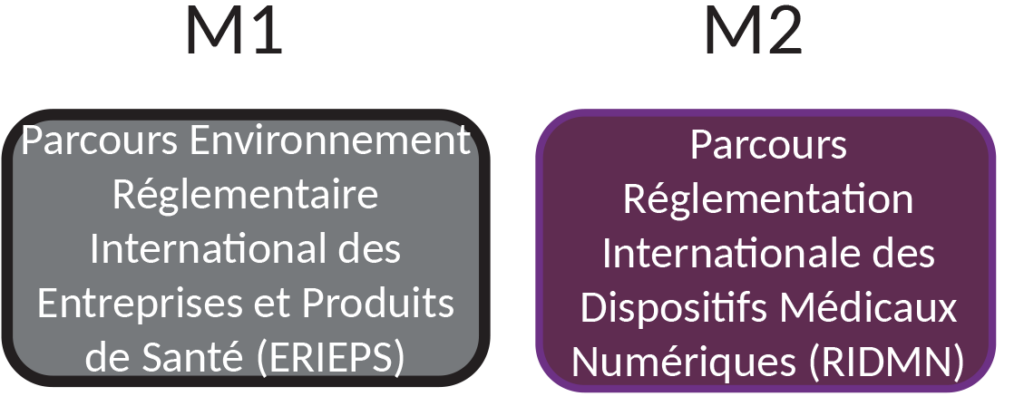
As early as the M1 ERIEPS course, students receive an introduction to digital health.
If students want to specialize in digital health in M2, they can follow the RIDMN pathway, which shares a common core with M2 ERIEPS.